Hello, everyone! This semester, I am writing a series of blog posts about the effects of ocean acidification on coral reefs. If you are unfamiliar with the concept of ocean acidification, please check out my last blog post, “When Air Meets Water: Carbon Dioxide And Ocean Acidification.” You will find an explanation of the ocean acidification process and an examination of the negative relationship between atmospheric carbon dioxide and calcium carbonate concentrations. Furthermore, the post shares projections for atmospheric carbon dioxide concentration, oceanic pH, and calcification rates, as well as describes how ocean acidification lowers aragonite (calcium carbonate) saturation and threatens reef building and reef organisms.
In this post, I will be expanding more upon the effects of ocean acidification on reef organisms. As mentioned in my last blog post, relative to pre-industrial rates, corals, calcifying macroalgae, and other reef-building organisms are expected to calcify 10-50% less by 2050.1 As a result, corals will become rarer, and reefs and reef organisms will experience more difficulty functioning.1 Furthermore, the decline in production and rise in dissolution of calcium carbonate will lessen breakwater effects that shield coastlines and generate habitats for mangroves, seagrass beds, and more.1
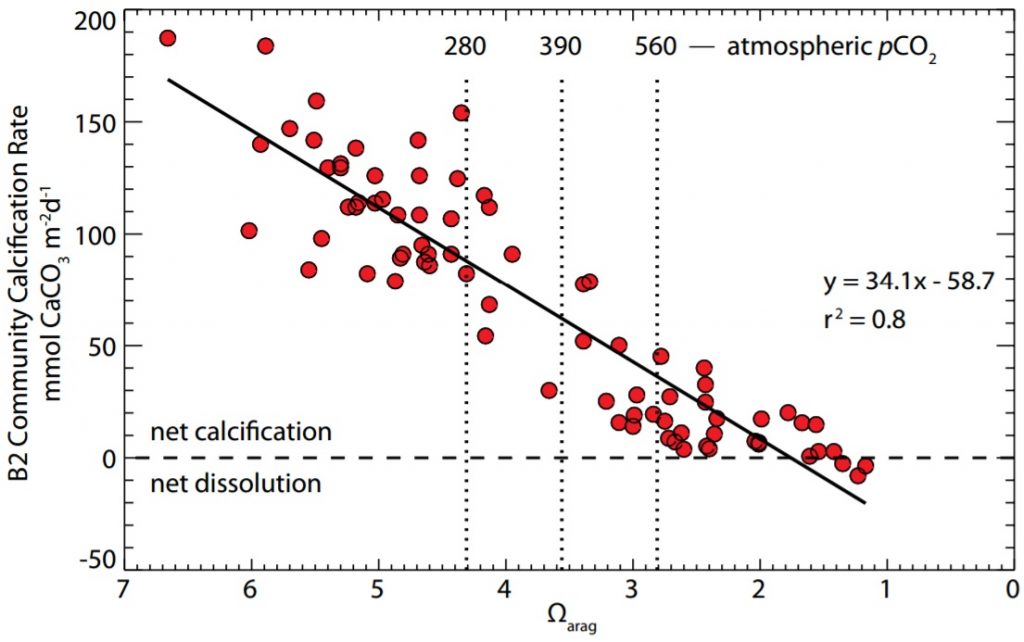
Figure 1. Coral community calcification rate changes in the Biosphere 2 coral reef mesocosm with respect to decreasing aragonite saturation state. Corresponding atmospheric pCO2 levels (ppm) and Ωarag values shown: 280 = pre-industrial, 390 = present, 560 = 2X pre-industrial. Shift from net calcification to net dissolution at Ωarag = 1-2. © Source: Kleypas et al.1
Ocean acidification decreases skeletal growth in reef-building corals and coralline algae, which likely cannot adapt to these changes, by lowering calcification rates.1 Figure 1 shows that as aragonite saturation states decreased, coral community calcification rates in the Biosphere 2 coral reef mesocosm also decreased. While calcification will increase if ocean acidification is reversed and aragonite saturation states increase, it is highly unlikely that ocean acidification can be reversed.1 (If so, then what? I will be writing about possible future steps in my next blog post!)
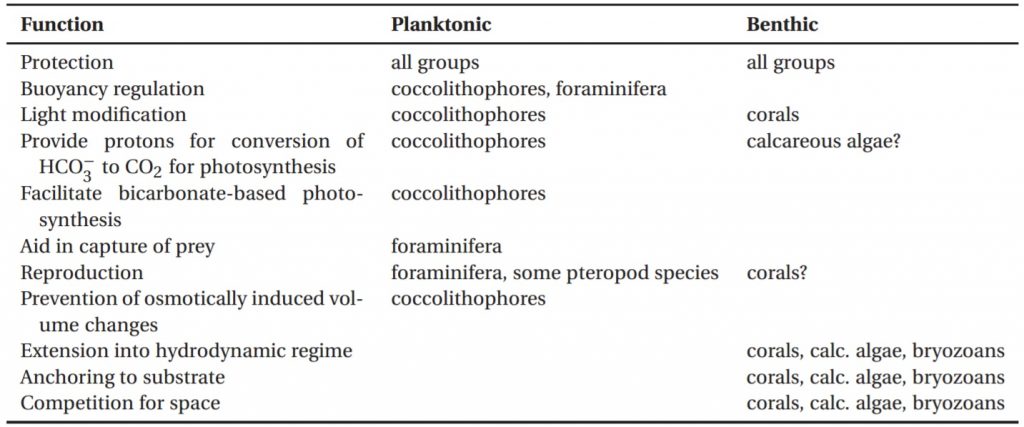
Calcium carbonate secretions from organisms help provide skeletal support, protection, and many other functions, as indicated in Table 1. Thus, reduced calcification may cause organisms to lose beneficial functions. For example, with lessened protection, a species may get a microbial infection or be subjected to predation.2 Alternatively, reduced ballast (balance) could make it difficult for a species to maintain its position in the water column.2 In corals and coralline algae, significant skeletal growth elevates the organism into better light (increased light gathering) and flow (more resilience against hydrodynamic forces) conditions, while slower or more fragile growth lowers reproductive success.2 For example, size (not age) determines reproductive maturity in Goniastrea aspera, and skeletal fragmentation in Acropora palmata reduces sexual reproduction potential.2
Reef-building corals may exhibit other harmful responses to reduced calcification as well. First, they may decrease their linear extension rate and skeletal density.3 Alternatively, they may maintain physical extension rates by reducing skeletal density, increasing risk of hydrodynamic damage and promoting bioerosion by parrotfish, which prefer to remove carbonates from lower-density substrates.3 Ultimately, coral reefs would have lower structural complexity, habitat quality, and diversity.3 Lastly, in response to reduced calcium carbonate saturation, corals may maintain skeletal growth and density by investing more energy in calcification.3 However, this diverts resources from reproduction, reducing larval output from reefs and making recolonization following disturbances difficult.3
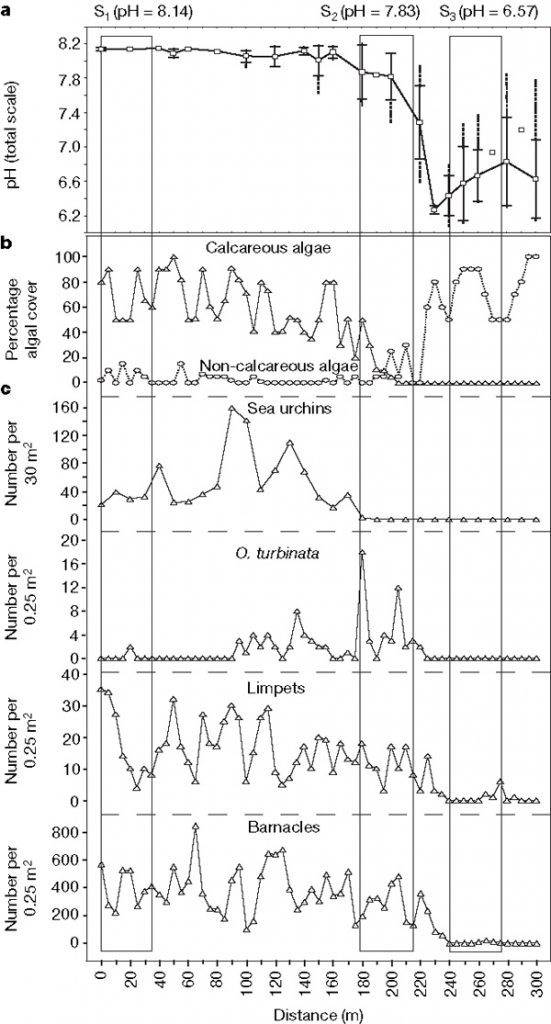
Figure 2. Variation in pH, algal cover, and species abundance at carbon dioxide vents south of Castello d’Aragonese from April 18 to May 9, 2007. (a) Mean s.d. (cross bars) and range (dotted lines) of pH. (b) Calcareous (triangles) and non-calcareous (circles) algal cover. (c) Sea urchin, O. turbinata, limpet, and barnacle abundance. © Source: Hall-Spencer et al.4
As mentioned previously, ocean acidification can alter ecosystem dynamics and reduce reef diversity. Figure 2 illustrates relationships between pH, algal cover, and species abundance that were determined using a volcanic carbon dioxide vent system. In Figure 2B, as pH drops, non-calcareous algae overtakes calcareous algae in cover dominance. In Figure 2C, sea urchins, which help maintain ecosystem complexity and stability,4 disappeared first (pH = 7.4-7.5) as the pH dropped. On the other hand, calcitic organisms like barnacles, which can close their rostral plates around supplies of ambient water,4 survived until pH dropped to 6.6. Finally, under pH 7.4, adult Opuntia turbinata and limpet gastropod shells are weakened, increasing risk of predation,3 as evidenced by the drop in gastropod abundance.
Ultimately, it is clear that reef organisms suffer from and are unable to effectively respond to ocean acidification. With time, the loss of certain functions in specific organisms can lead to a general decrease in reef diversity and structural complexity. Without intervention, coral reefs are destined to lose their rich vibrancy and become dull, empty piles of rubble and dead coral. So think long and hard about how you can help slow down the ocean acidification process! (And if you have any trouble, I will help you out in my next post. :))
References:
- Kleypas, Joan A., and Kimberly K. Yates. “Coral reefs and ocean acidification.” Oceanography (2009).
- Kleypas, Joan A., et al. “Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research.” Report of a workshop held. Vol. 18. 2005.
- Hoegh-Guldberg, Ove, et al. “Coral reefs under rapid climate change and ocean acidification.” science 318.5857 (2007): 1737-1742.
- Hall-Spencer, Jason M., et al. “Volcanic carbon dioxide vents show ecosystem effects of ocean acidification.” Nature 454.7200 (2008): 96-99.