Hi, everyone! This is my third and final post about the effects of ocean acidification on coral reefs. In my first and second posts, I explored the concept of ocean acidification and how it affects reef organisms. In this post, I will be going over some steps that can be taken to fight against the acidification process. Feel free to check out my previous posts for a better understanding of why ocean acidification is worth addressing!
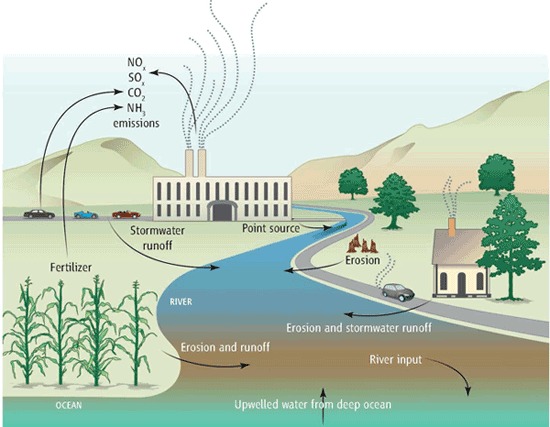
Figure 1. Ocean acidification sources, with an emphasis on local contributors. © Source: Kelly et al.2
In my last post, I mentioned that ocean acidification is highly unlikely to be reversed.1 While this is (currently) true, several strategies demonstrate significant mitigation potential. Before we explore these ideas, I want to emphasize that although ocean acidification is a global issue, it disproportionately affects local coasts and communities.2 Figure 1 illustrates several major local sources that contribute to coastal acidification.
Considering this and the difficulty of gaining traction through national and global efforts, smaller-scale measures are becoming increasingly crucial. Local and state governments within the United States are able and motivated to modulate emissions, runoff, and land-use patterns through zoning and permitting.2 Some states have already limited residential runoff, reducing water contamination, algal blooms, and pollutants in local coasts.2
Another local approach for combating acidification involves controlling coastal erosion and reducing nutrient and sediment loading of water by increasing vegetation cover.2 Additionally, anti-sprawl land-use plans can reduce vehicle-miles traveled and impermeable surface cover, limiting emissions and runoff.2 Furthermore, enforcement of federal emissions limits for nitrogen and sulfur oxide by local and state governments would have immediate effects, as these pollutants have short atmospheric residence times, quickly falling into the water.2 Finally, capitalization of Clean Water Act (federal) funding by states for stormwater surge prevention, coastal and riparian buffers, intact wetlands, and improved onsite water treatment facilities would limit runoff and associated pollutants.2
Now, am I missing something? You might be thinking, “Isn’t the obvious answer to just reduce atmospheric CO2 levels?” Well, it’s not as easy as it seems. While essential, reducing CO2 emissions may not be enough, given our limited time frame.3 We would have to reverse the current 3% annual increase in CO2 emissions by 2020 to keep atmospheric CO2 levels below 550 parts per million by volume, and even greater reductions would be needed to maintain favorable coral growth conditions.5
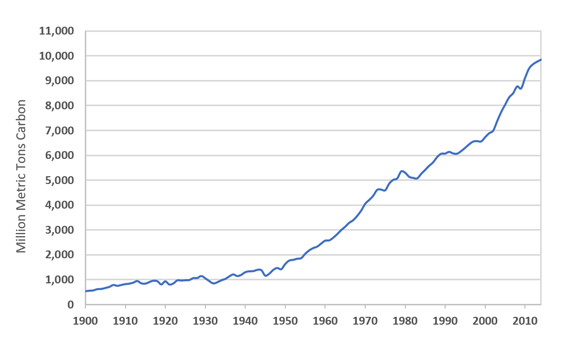
Figure 2 depicts global trends in anthropogenic carbon dioxide emissions. Because atmospheric CO2 levels continue to rise despite growing awareness of the breadth and potential cost of ocean acidification, we must look to alternative solutions.3 Furthermore, even if emissions ceased, which is pretty much out of the question, excess CO2 in the ocean-atmosphere system would persist for several millennia.3
Still, I do want to bring up one emission-related measure that should be taken: Atmospheric CO2 is often lumped with other greenhouse gases when considering climate protection. However, as the core pollutant involved in ocean acidification, it would be better to consider it separately and to limit its emissions regardless of other greenhouse gas regulations.5
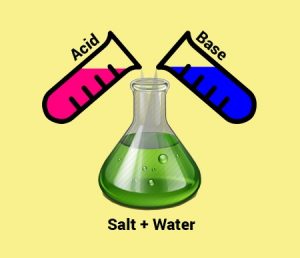
Figure 3. Neutralization reaction. This image visualizes a general neutralization in which an acid and base combine to form a salt and water. © Source: TutorVista.com6
Returning to alternative ocean acidification combat methods, one idea is to neutralize CO2 acidity by adding base minerals to the ocean.5 Figure 3 illustrates a general neutralization reaction. While base mineral reactions in surface waters are normally slow, reacting seawater and carbonate minerals with concentrated CO2 in flue gas or other waste streams could speed them up.3 Ultimately, this would consume waste CO2, avoid emissions, generate seawater alkalinity, increase calcium carbonate saturation states, and help reinforce marine biological shells at a local scale.3 In the long run, optimal application of 0.48 Gt of CaCO3 a-1 could absorb atmospheric CO2 at a rate of 600 Mt CO2 a-1 after 50 years.7
Another approach, although ineffective at mitigating ocean acidification itself, increases resilience against it. Compared to their wild-type counterparts, Saccostrea glomerata reared in increased CO2 withstand acid stress more effectively.3 Similar observations have been made in studies on Acropora hyacinthus under elevated temperatures.8 However, although many options exist, few ideas have been studied past the conceptual or laboratory stage.3 To discover the safest and most cost-effective solutions, further research and in situ testing is necessary, so next time you donate to a cause, consider helping fund a project related to one of these ideas!
References:
- Kleypas, Joan A., and Kimberly K. Yates. “Coral reefs and ocean acidification.” Oceanography (2009).
- Kelly, Ryan P., et al. “Mitigating local causes of ocean acidification with existing laws.” Science 332.6033 (2011): 1036-1037.
- Rau, Greg H., Elizabeth L. McLeod, and Ove Hoegh-Guldberg. “The need for new ocean conservation strategies in a high-carbon dioxide world.” Nature Climate Change 2.10 (2012): 720-724.
- Marland, Gregg, et al. “Global, regional, and national fossil fuel CO2 emissions.” Trends: A compendium of data on global change (2003): 34-43.
- Logan, Cheryl A. “A review of ocean acidification and America’s response.” Bioscience 60.10 (2010): 819-828.
- “Acid-Base Properties of Salts.” TutorVista.com. N.p., n.d. Web. 20 Apr. 2017.
- Harvey, L. D. D. “Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions.” Journal of Geophysical Research: Oceans 113.C4 (2008).
- Mascarelli, Amanda. “Designer reefs.” Nature 508.7497 (2014): 444.