Carbon dioxide (CO2) is not just in the air we breathe; it is also in oceans that harbor coral reefs. Unfortunately, due to increasing atmospheric carbon dioxide concentrations, many reefs are threatened by ocean acidification, which will be the focus of my blog posts. This issue is important because in addition to ecological diversity, coral reefs support economies through tourism and fisheries, they provide coastal protection, and they serve as sources of building materials and new biochemical compounds.1
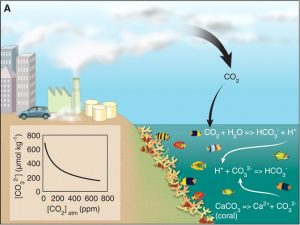
Figure 1. Negative correlation between atmospheric CO2 levels and coral calcification due to ocean acidification. This image shows how atmospheric CO2 becomes bicarbonate ions and protons, decreasing oceanic pH and carbonate concentrations. © Source: Hoegh-Guldberg et al.1
During the 20th century, ocean acidity decreased by 0.1 pH units.1 (While that sounds insignificant, pH is on a log scale!) From 2000 to 2006, ~25% of CO2 emitted from anthropogenic sources entered the ocean.1 There, it reacted with water to form carbonic acid, which dissociates into bicarbonate ions and protons.1 Bicarbonate ions (CO32-) and protons react with carbonate ions to form more bicarbonate ions, reducing carbonate availability for calcifiers like corals.1 Figure 1 illustrates the process of ocean acidification and the relationship between CO2 and CO32- levels.
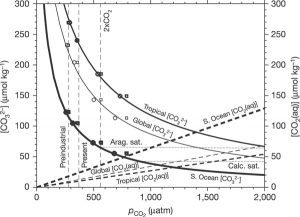
Figure 2. Average surface water CO32- and CO2 levels in the tropical, Southern, and global ocean. This image shows key carbonate chemistry variables as a function of pCO2. © Source: Orr et al.2
Figure 2 also depicts the relationship between atmospheric CO2 and CO32- levels, but in more detail than Figure 1, which does not include aqueous CO2 levels or time or site-specific information. Furthermore, Figure 2 projects undersaturation with respect to aragonite, a metastable form of calcium carbonate, and calcite.2
Atmospheric CO2 concentrations surpassed 380 ppm (which is 80 ppm higher than maximum values during the past 740,000 years) a decade ago and are expected to exceed 500 ppm by 2050-2100.1 Anthropogenic CO2 is estimated to have increased hydrogen ion concentrations in surface waters by 30% since the 20th century, and seawater pH may drop by 0.5 units by 2100.3 Relative to pre-industrial rates, corals, calcifying macroalgae, and other reef-building organisms are expected to calcify 10-50% less by 2050.4

Figure 3. Effects of ocean acidification on marine organisms. (a) P. oceanica with (pH 8.2) and (b) without (pH 7.6) Corallinaceae growth; (c) O. turbinate with (pH 8.2) and (d) without (pH 7.3) intact periostracum; (e) P. caerulea and (f) H. trunculus with eroded, pitted shells (pH 7.4). © Source: Hall-Spencer et al.3
Under these conditions, calcium carbonate (CaCO3) levels will drop below saturation, compromising carbonate accretion and making it difficult to maintain the structural complexity of reefs.5 As a result, corals will become rarer, lowering reef biodiversity,1 and reefs and reef organisms will experience more difficulty functioning.4 Figure 3 shows how some reef organisms are affected by ocean acidification. The decline in production and rise in dissolution of CaCO3 will also lessen breakwater effects that shield coastlines and generate habitats for mangroves, seagrass beds, and more.4
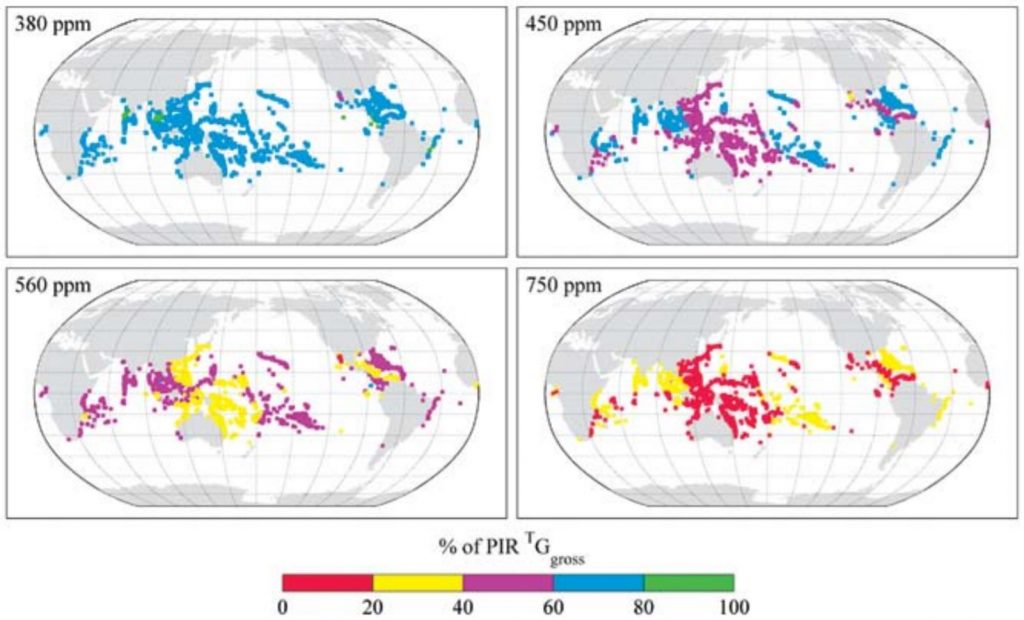
Figure 4. Predicted effect of atmospheric CO2 levels on reef building worldwide. Values in this image are expressed as a percentage of pre-industrial (atmospheric CO2 concentration = 280 ppm) calcification rates. PIR = pre-industrial rate; TGgross = temperature-dependent gross calcification. © Source: Kleypas et al.4
As ocean acidification proceeds, reefs around the world will shift into net erosional states, with slow-growing reefs transitioning first.5 Figure 4 illustrates the expected progression of reduced reef calcification with respect to increasing atmospheric CO2 concentrations. With CO2 emissions on the rise, we are heading down a dark, dark path. If steps are not taken to effectively combat ocean acidification, the colorful and diverse settings of movies like Finding Nemo may eventually become unrecognizable.
References:
- Hoegh-Guldberg, Ove, et al. “Coral reefs under rapid climate change and ocean acidification.” science 318.5857 (2007): 1737-1742.
- Orr, James C., et al. “Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.” Nature 437.7059 (2005): 681-686.
- Hall-Spencer, Jason M., et al. “Volcanic carbon dioxide vents show ecosystem effects of ocean acidification.” Nature 454.7200 (2008): 96-99.
- Kleypas, Joan A., and Kimberly K. Yates. “Coral reefs and ocean acidification.” Oceanography (2009).
- Kleypas, Joan A., et al. “Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research.” Report of a workshop held. Vol. 18. 2005.

